how to draw molecular orbital diagram of n2
The purpose of MO theory is to fill in the gap for some. Orbitals represented by are antibonding orbitals and the orbitals without are bonding orbitals.

Write The Molecular Orbital Diagram Of N2 And Calculate Their Bond Order Chemistry Topperlearning Com Qbqjy
Draw the molecular orbital diagram for N2 and calculate the bond order.
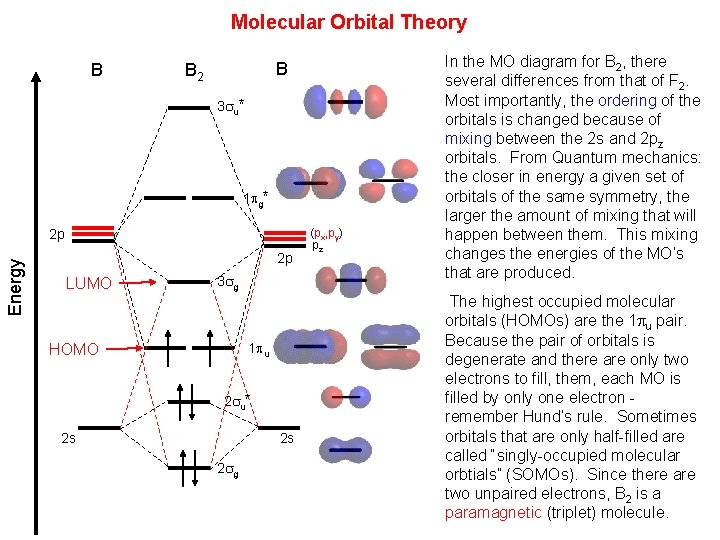
. In the Lewis structure of the N2 molecule there is a formation of a triple covalent bond represented by three lines between two atoms of Nitrogen. If we build the MO diagram for N_2 it looks like this. MO Diagrams for Elements Li2 through Ne2.
In contrast to VSEPR and valence bond theory which describe bonding in terms of atomic orbitals molecular orbital theory visualizes bonding in relation to molecular orbitals which are orbitals that surround the entire molecule. The Bond Order Formula can be defined as half of the difference between the number of electrons in bonding orbitals and antibonding orbitals. Molecular orbital diagram for nitrogen gas N2Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s2sigma2s2pi2p4sigma2p2Bond Or.
Dont memorize Li2 through N2. Bond order can be calculated by the formula. Draw the MO diagram for N2.
I think you can safely assume to start off with the molecular orbital diagram of the Nitrite anion NO₂ and then remove an electron from it. Show activity on this post. Part A Complete the MO energy diagram for the N2 ion by dragging the electrons Electron with spin up in the figure given belowMO.
The formal bond order of N 2 is 3 from about one σ- bond and two π- bonds. Given mass of Al NO33 155 g Mass of water solvent 672 g 0672 kg Kb for water 052Cm. Even rather simple molecular orbital MO theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed from N2 O2 F2 Ne2 the complexity of the molecular orbitals develop in two ways.
In this case the difference is the H-X-H bond angle which decreases from o to 90 o Molecular Orbital Theory. For N X 2 the orbitals in increasing energy are. Bond order bonding electrons - antibonding electrons 2.
The last electron is in fact in the 2b_1 antibonding mo so the bond order of no has decreased by 12 relative to no or. What Is The Molecular Orbital Diagram For No Quora. Molecular orbital diagram of N 2 is shown below.
1 If N b Nathe molecule is stable because greater number of. Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na. In the molecular orbital diagram for the molecular ion N 2 the number of electrons in the σ 2p molecular orbital is.
EXAMINENTIVNOJ Draw the products of. They werent drawn that way on this diagram but they should be. Molecular orbital diagram of no.
Molecular orbitals for larger molecules 1. VSEPR model assumes that molecular geometry minimizes the. Predict whether S is positive or negative for the reaction.
Relationship between electronic configuration and Molecular behaviour. The leftover two 2p orbitals become two π bonds and electrons making a pair between the nitrogen atoms will make a sigma bond. Abstract TLDR Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds.
Procedure to draw the molecular orbital diagram of CN. Molecular Orbital MO Theory is the final theory pertaining to the bonding between molecules. The molecular orbital diagram for Nitrogen dioxide NO₂ should loo.
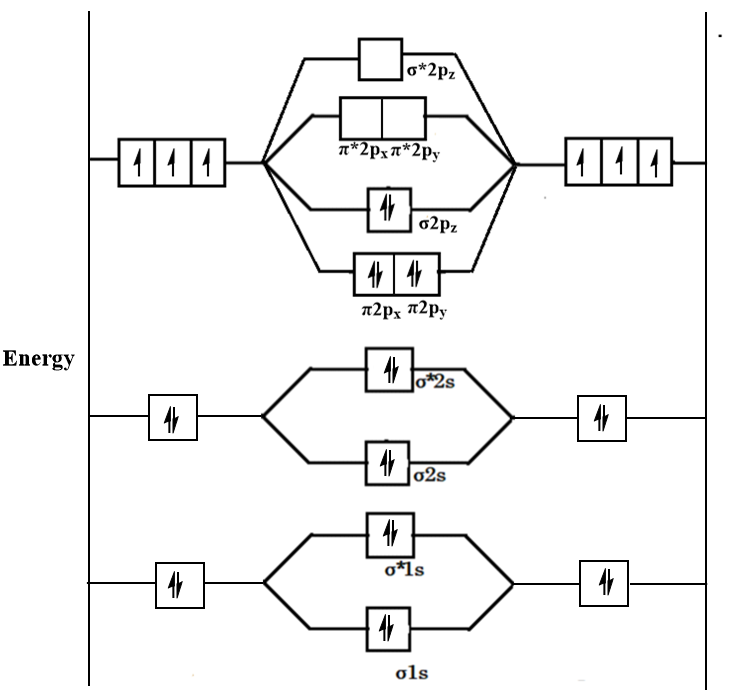
Molecular orbital theory explanation. Its most important property is its lack of reactivity so that as the principal diluent it can mitigate the dangerous properties of O 2 in air. Bond order 1 2 Number of electrons in BMO Number of electrons in ABMO Bond order 1 2 8 2 Bond order 1 2 6 Bond order 3.
N2 g O2 g 2NO g. The other is for AFTER nitrogen start. Number of electrons in antibonding orbitals.
A Number of electrons in bonding molecular orbitals. Anyways for the electron configurations you would use a notation like the above. N 2 is a very stable 10-valence-electron molecule isoelectronic with CO and with CN.
Does this diagram predict that N2 isdiamagneticlor paramagnetic. We assume that orbital order is the same as that for N2. Number of electrons in bonding orbitals.
Does this diagram predict that N2 isdiamagneticlor paramagnetic. O2 kk σ2s 2 σ2s 2 π2p x 2 π2p y 2 π2p x 1 π2p y 1. Electronic configuration of co molecule is.
Diagram for N2Molecular orbital diagram - Wikipedia. G means gerade or even symmetry upon inversion and u means ungerade or odd symmetry. Bond order formula is given as below.
I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. Relationship between electronic configuration and Molecular behaviour. Draw the molecular orbital diagram for N2 and calculate the bond order.
Which of the follwing do not exist He2 HeH Be2 A. What will be the molecular orbital diagram for nitrite ion. There are two MO diagrams you need to memorize for diatoms N2 O2 Ne2 etcOne is for the elements up to Nitrogen.
Assign x y z coordinates z axis is principal axis. Determine the boiling point of the solution if 155g of Al NO33 is dissolved in 672g of water. This interaction introduces an element of s-p mixing or hybridization into the molecular orbital theory.
B Number of electrons in antibonding molecular orbitals. 1 Stability of molecules in terms of bonding and antibonding electrons. The molecular orbital diagram MO Diagram of N.
This picture shows the molecular orbital diagram of N 2. Figure A partial molecular orbital energy-level diagram for the HF molecule. Write the molecular orbital diagram of N2 and calculate their bond order why nitrogen have different structure of molecular orbital theory An atomic orbital is monocentric while a molecular orbital is polycentric.
So the formula to find bond order is. I Structure of N 2. Bond order 2nb na 284 2.
O2 kk σ2s 2 σ2s 2 π2p x 2 π2p y 2 π2p x 1 π2p y 1. The bond order is Figure The molecular orbital energy-level diagram for both the NO and CN-ions. Draw the mo diagram for n2.
There are two MO diagrams you need to memorize for diatoms N2 O2 Ne2 etcOne is for the elements up to Nitrogen. First though notice that the p orbitals are supposed to be degenerate. Give method and find hybridisation and shape of.
- N 2 molecules are diamagnetic with no unpaired electrons. σ 1 s σ 1 s σ 2 s σ 2 s π 2 p x π 2 p y σ 2 p z π 2 p x π 2 p y σ 2 p z.

How Does The Lewis Structure Of N2 Related To The Molecular Orbital Bonding Model Quora
Draw The Molecular Orbital Diagram Of N2 And Calculate Class 12 Chemistry Cbse

Easiest Trick To Draw Molecular Orbital Diagram Chemistry Chemistry Molecular Chemistry Notes

Molecular Orbital Theory Or When Electrons Dont Like

How To Draw The Molecular Orbital Diagram Of N2 Bond Order Of Nitrogen Molecule Chemistry Youtube

Orbitals What Is The Origin Of The Differences Between The Mo Schemes Of O And N Chemistry Stack Exchange

8 5 Molecular Orbital Theory Chemistry

Draw The Molecular Orbital Energy Level Diagram Of N2 Molecules
Draw The Molecular Orbital Diagram Of N2 Also Find Its Bond Order And Magnetic Character Chemistry Topperlearning Com 4s4p942zz

Draw A Molecular Orbital Diagram Of N2 Or O2 With Magnetic Class 11 Chemistry Cbse

Molecular Orbital Diagram Of B2 C2 And N2 Molecules Youtube

Molecular Orbital Mo Diagram For N2 Youtube

Molecular Orbitals Chemistry Classroom Nomenclature Chemistry Chemistry Study Guide

Mo Diagram Of Nitrogen Molecule And Its Ions Youtube

Molecular Orbital Mo Diagram Of N2 Youtube
Explain The Mo Diagram For No Molecule Sarthaks Econnect Largest Online Education Community

Introduction To Molecular Orbital Theory Chemistry Education Chemical Science Physical Chemistry